
ISO 13485 CERTIFICATION – MEDICAL DEVICE QUALITY MANAGEMENT SYSTEM
Upholding superior quality in medical device production & healthcare practices, assure compliance with international standards: ISO 13485 Quality Management System
What is ISO 13485 Certification?
The ISO 13485 is the medical devices quality management system. Medical devices are one of the most important parts of the medical system. It’s impossible to move ahead without them. However, the quality of the devices always creates a major concern.
The standard specifies all the directives and requirements needed to maintain quality. In many countries around the world, only ISO 13485 does not suffice for selling a medical device. It may also require a clearance from the local body.
The latest version of the ISO 13485 Certification QMS is the ISO 13485:2016. This describes the current QMS requirements for organizations related to medical devices.
Besides, we have widespread recognition in the areas of ISO 13485 Quality Management System in UAE – Abu Dhabi, Dubai, Sharjah, Ajman, Umm Al Quwain, Ras Al Khaimah, and Fujairah.
ISO 13485 Certification in Brief:
ISO 13485:2016 is based on the ISO 9001 process model approach and is a management system standard explicitly produced for medical devices. Its essential goal is to work with blended medical gadgets services purpose. The standard contains explicit necessities for the production, establishment, and adjusting of devices and calls for:
- Management of a Quality Management System with a few upgrades
- Risk Management way to deal with item improvement and item acknowledgment
- Approval of process
- Compliance with legal and regulatory prerequisites
- Powerful product detectability and auditing system
This standard permits organizations to lessen security and legal threats while establishing more affordable workplaces. As a globally recognized norm of value and security for medical device production, having an ISO 13485 certificate assists organizations with getting perceived as additional respectable, dependable suppliers. The latest edition of ISO 13485 is seen at regular intervals and revised by the new requirements and necessities of the industrial aspects.
ISO 13485 has seen a 33.1% increment in overall certificates in 2020, showing the development and significance of UKAS-licensed certificates as of late.
Importance of ISO 13485 Standard Certification :
The population of the world is growing. With this, there is an increase in health-related issues. Public health, these days, is one of the top priorities for the government. For this, a large number of medical facilities have been set up in every country around the world. The government of almost every country around the world has put the utmost importance on public health.
ISO 13485 certificat ensures a QMS for organizations related to medical devices and it makes sure that the operations in the organization are compliant and adheres to the directives, requirements and regulations.
Benefits of ISO 13485 Certification:
The benefits of ISO 13485 are not for the UAE alone, but Ascent also covers the whole of Middle East regions. It can be seen further in the long run for an organization or a firm that accepts the quality standard for medical devices and services.
It can be considered as a means for excelling in the market by providing qualified services of ISO 13485 Quality Management System in UAE, Oman, & Sharjah.
ISO 13485 Certification benefits include:
- Meeting the requirements– The standard ensures that an organization adhered to the directives set by it and also meets the desired requirements regarding product quality.
- Enhanced reliability– It enhances the credibility and trustworthiness of an organization.
- Product performance– It increases the performance of the products to meet the standard of the ISO 13485 in Oman.
- Competition– The certification in Oman and Saudi Arabia gives an organization an advantage in competition by increasing business stability. It gives the certified organization an edge over non-certified ones.
- Manpower– This creates a proper system to monitor the performance of the personnel working within the organization.
- Procedural ease – The procedure of certification is very simple and lucid. For that, the certification becomes a lot easier.
- Customer satisfaction – A business that can work without any kind of disruptions can always bring more customer satisfaction through smooth operations.
- Brand value – Brand value is a key to every business. Operating without any potential disruptions often sets a high brand value for a business body.
Which Organizations Are Eligible to Go for ISO 13485?
A lot of organizations require ISO 13485 Quality Management System in Dubai. It includes a manufacturer, distributor, supplier, retailer of medical devices or any other organization that deals in medical devices. In some countries like Oman, and Saudi Arabia, we have made rendering ISO 13485 possible.
ISO 13485 is used mostly by the internal and external parties, such as the certification bodies. These bodies are involved in the installation, design, production, and maintenance of medical devices, and other such servicing.
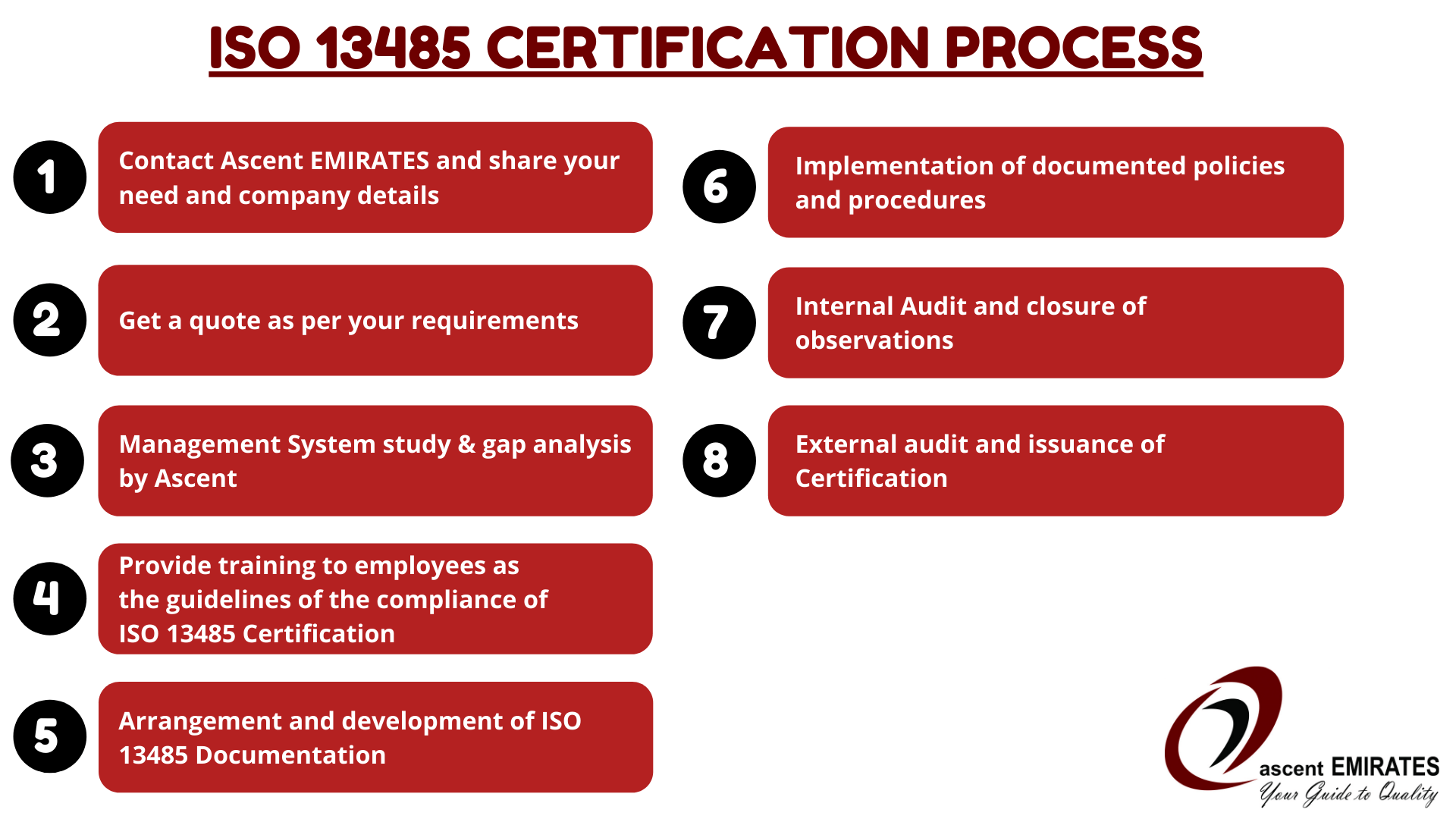
Requirements of ISO 13485 Certification:
The ISO 13485 Quality Management System in UAE has a set of directives, guidelines, and requirements.
Following these requirements enables an organization to successfully implement the standard.
- The plan and need must be determined.
- Identification of quality management systems in the medical process and procedures.
- Prepare all the documents that are required to fulfill the needs of the standard.
- Regular maintenance of medical devices.
- Timely sanitization of devices.
- A yearly internal audit of an organization.
Details of the roles the organization undertook to match the applicable regulatory requirements. After satisfying all legal requirements and guidelines, you will pass the process to manufacture medical equipment at your premises.
Strength of Ascent EMIRATES
- Help you in assuring that your product quality is compliant to the standard and acquire the certificate.
- Helps to acquire the certificate without any difficulties and with an easy documentation process. Ascent will help you to have the certification done in the simplest way.
- Has qualified ISO 13485 lead auditors with a professional attitude to handle your various problems related to conformity and certification of compliance.
- Works 24*7 for their customers and clients with a simple motto, “No extra cost than the offered fees” to an organization.
- Explains the importance and implementation of the standard.
- Trains and guides you throughout the process to achieve the certification.
- There is no compromise when it comes to the quality of your medical devices, that is why you can trust Ascent EMIRATES by providing reliable and innovative solutions to your problems.
Get in touch with Ascent EMIRATES, to have the best ISO 13485 Quality Management System in UAE, Oman, & Saudi Arabia today! So, connect with our expert advisory team @ uaeoffice@ascentworld.com to learn more. Dial : +971-4-4558490 to avail Best Offers.
Frequently Asked Question
Q1. What is the duration required to avail of ISO 12485 Certification?
The time it takes to obtain the ISO 13485 Certification in Oman depends on the size of the organization. In the case of a smaller organization, it can be done within 3–4 months, whereas in the case of a larger organization, it can take from 8 months up to a couple of years.
Q2. How much you will pay for the ISO 13485 Certificate?
The pricing of ISO 13485 Quality Management System in Dubai and Abu Dhabi may require a little stuff such as the type of the device. With the certification, the products may become a little more expensive than their usual price. ISO 13485 gives reliable quality, is very crucial for safety, and takes care of customers in different aspects.
Q3. What is the total time required for obtaining an ISO 13485 Certificate?
Well, the ISO 13485 Quality Management System process in Sharjah depends on different factors such as the strength of your organization, documentation, approvals, auditing, and other charities. Give or take, you will get your certificate within 3 to 6 months.
Q4. How do you define class 1, 2, and 3 medical devices?
As per the FDA, medical devices are classified into three different categories.
They are as follows:
- CAT 1 / CLASS I: Devices to use with general control at low risk.
- CAT 2 / CLASS II: Medical devices available in general store at moderate risk.
- CAT 3 / CLASS III: Medical devices with high risk for sustaining life.


